高碘酸鈉氧化鄰烷基羥基苯酚得到 6,6-螺-2,4-環己二烯酮化合物,接著自發通過Diels–Alder加成二聚的反應。此反應也被稱為Adler-Singh反應。
反應機理
高碘酸鈉通過與底物的酚羥基和烷基羥基配位后氧化,得到6,6-螺-2,4-環己二烯酮化合物,接著自身進行D-A加成反應得到產物。
反應實例

NaIO4 (47 g,0.22 mol) inwater (1 L)was addedtoa stirred solution of 2-hydroxybenzyl alcohol 1 (n = 1, 24.83 g, 0.2 mol) in water (1.5 L).After 10 min, colorless crystals appear.The mixture was kept for 24 h at 4 C in the dark. The crystalline product was filtered, washed (water) and dried in vacuum over P2O5 to afford 18.05 g of 3 (74%), mp 194–195 C.
【Adler E, Acta Chem Scand, 1971, 25, 2055】
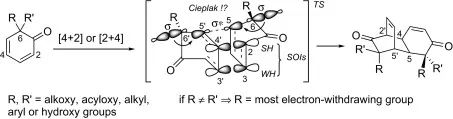
【Castet F, Quideau S, Tetrahedron, 2007, 63, 6493】

【Singh V, Acc Chem Res, 1999, 32, 324】
相關文獻
1 Adler E, Acta Chem Scand, 1959, 13 505
2 Adler E, Acta Chem Scand, 1960, 14 1261, 1580
3 Adler E, Acta Chem Scand, 1962, 16 529
4 Adler E, Acta Chem Scand, 1971, 25 2055
5 Singh V, J Chem Soc Chem Comm, 1992, 1212
6 Waldmann H, Tet Lett, 1996, 37, 3833
7 Singh V, Acc Chem Res, 1999, 32, 324
8 Castet F, Quideau S, Tetrahedron, 2007, 63, 6493
9 Singh V, Org Biomol Chem, 2010, 8 ,4472